Effects of Eribulin on Microtubule Binding and Dynamic Instability Are Strengthened in the Absence of the βIII Tubulin Isotype
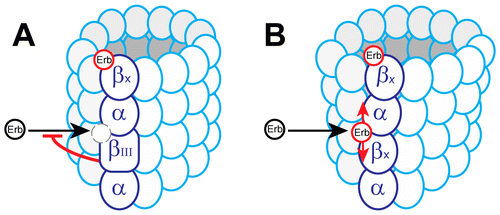
Possible model for eribulin binding to microtubule plus ends in the presence or absence of βIII tubulin. (A). Eribulin (Erb) bound to the plus tip of a microtubule is shown as a red circle, unbound eribulin is shown as a black circle. Potential eribulin binding site between tubulin dimers (dashed circle) is sterically blocked by the rigid conformation induced by βIII tubulin. βx denotes any β tubulin isotype other than βIII (βI, βIII, βIV). (B). In the absence of βIII tubulin, the microtubule end is less rigid due to βx isotypes allowing for greater flexibility at the plus tip. Eribulin is able to bind both its terminal binding sites and to internal sites between tubulin dimers, increasing the maximum stoichiometry. When bound to an interdimer site, eribulin strengthens longitudinal tubulin-tubulln interactions (red arrows), stabilizing the plus end by suppressing rate and extent of shortening and the catastrophe frequency.
Leslie Wilson*†, Manu Lopus‡, Herbert P. Miller†, Olga Azarenko†, Stephen Riffle†, Jennifer A. Smith†, and Mary Ann Jordan†
† Neuroscience Research Institute, University of California, Santa Barbara, Santa Barbara, California 93106, United States
‡ Experimental Cancer Therapeutics and Chemical Biology, UM-DAE Centre for Excellence in Basic Sciences, Mumbai, India
Background
Eribulin mesylate (Halaven) is a microtubule-targeted anticancer drug used to treat patients with metastatic breast cancer who have previously received a taxane and an anthracycline. It binds at the plus ends of microtubules and has been shown to suppress plus end growth selectively. Because the class III β tubulin isotype is associated with resistance to microtubule targeting drugs, we sought to determine how βIII tubulin might mechanistically influence the effects of eribulin on microtubules.
Results
We found that while [3H]eribulin bound to bovine brain soluble tubulin depleted of βIII tubulin in a manner similar to that of unfractionated tubulin, it bound to plus ends of microtubules that were depleted of βIII-depleted tubulin with a maximal stoichiometry (20 ± 3 molecules per microtubule) higher than that of unfractionated microtubules (9 ± 2 molecules per microtubule). In addition, eribulin suppressed the dynamic instability behavior of βIII-depleted microtubules more strongly than and in a manner different from that of microtubules containing βIII tubulin. Specifically, with βIII tubulin present in the microtubules, 100 nM eribulin suppressed the growth rate by 32% and marginally reduced the catastrophe frequency (by 17%) but did not modulate the rescue frequency. However, in the absence of βIII tubulin, eribulin not only reduced the growth rate but also strongly reduced the shortening rate (by 43%) and the catastrophe and the rescue frequencies (by 49 and 32%, respectively).
Conclusion
When present in microtubules, βIII tubulin substantially weakens the effects of eribulin.